If your study materials are ready for a complete IRB review, you will need to submit an amendment request to your prescreening protocol.
Find the IRB Protocol
- At the top of the screen, use the IRB menu to switch to different screens, including Search IRB Protocols.
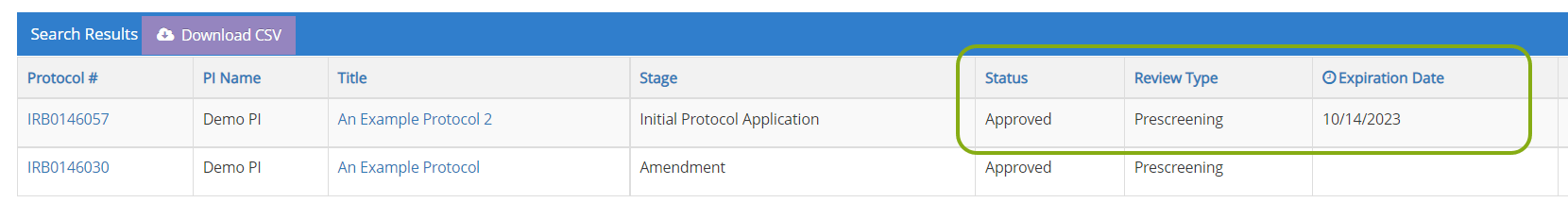
- Scroll down the page to the Search Results. You should see a list of protocols in which you are listed as part of the research team.
- You can find information such as the status of your protocol, review type, and expiration date directly in the search results.
- Locate the IRB protocol that needs to be amended. To open it click on the edit button, protocol ID, or title.
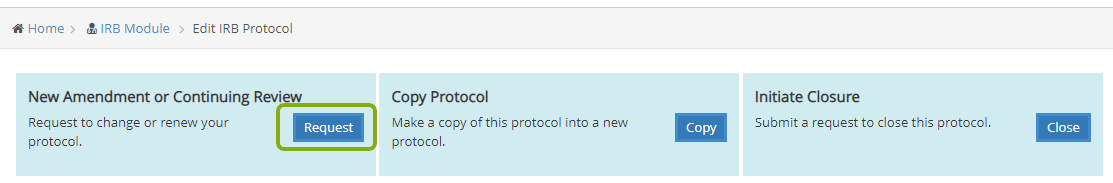
- Once your protocol opens, you will see 3 large blue boxes at the top of the protocol. Locate “New Amendment or Continuing Review” and click the Request button to start the amendment process. Note: If you don’t see these 3 blue boxes, it means you are listed as a research team member on the protocol but do not have permission to edit the protocol. If you need access to edit the protocol, please have your PI send an email to rass@research.cornell.edu requesting your permissions be updated for that protocol.
Amendment Form
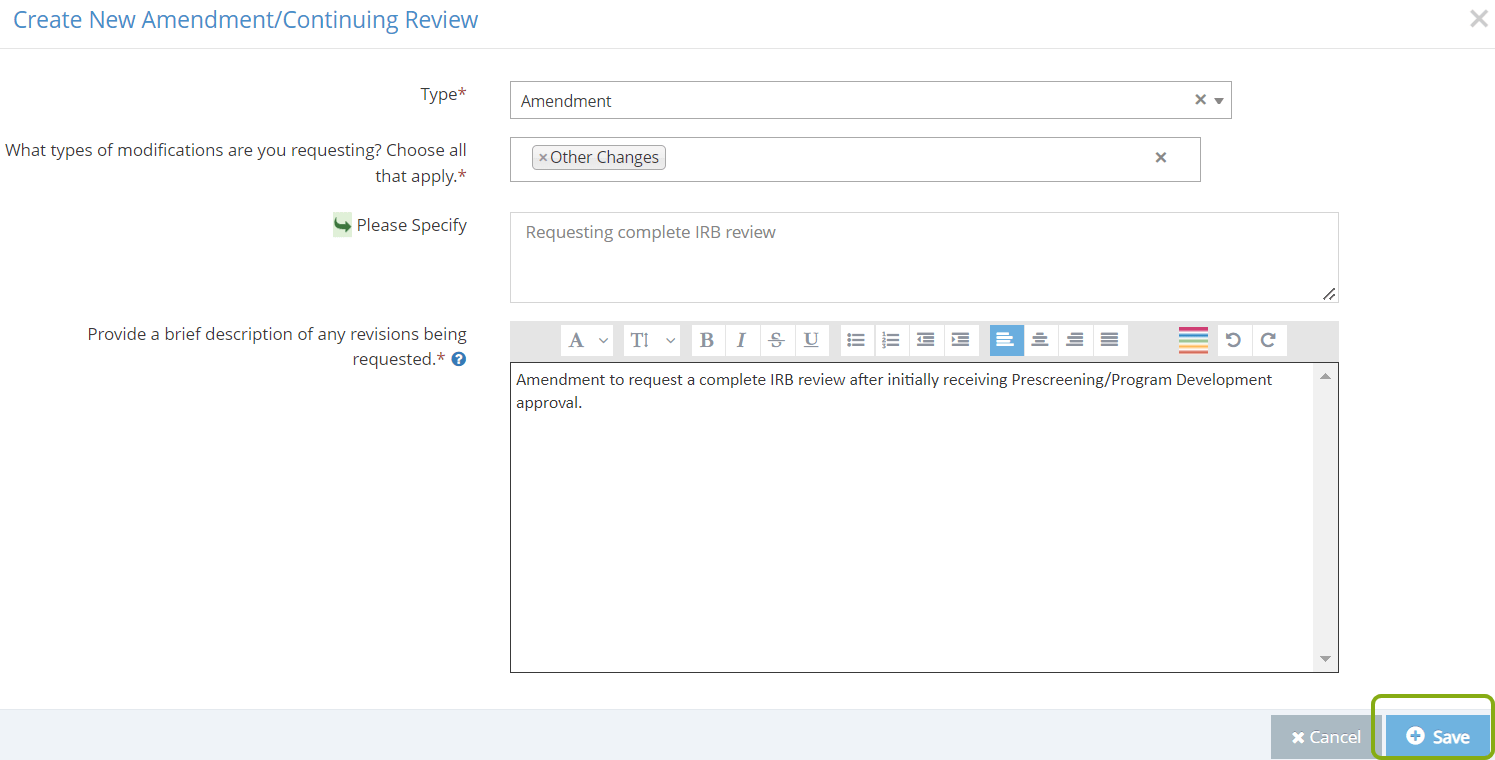
- Select the type of Amendment. The choices are Amendment, Continuing Review, or Amendment. Select Amendment.
- Select the types of modifications that you are requesting for your protocol. If you are requesting multiple types of changes, be sure to select each type.
- Provide a brief description of any changes you are requesting for your protocol. For example, that you are amending to request a complete IRB review.
- Click Save and RASS will unlock your protocol for editing.
Make Changes and Submit for Review
- Make the necessary changes and edits to your protocol. You can use the Panel Shortcuts on the left to get to the specific area of your protocol that require changes.
- To start, go to the Review Type Determination section of the Protocol.
- Change the answer to Are you requesting an IRB determination for a human participant research project for which the protocol is not yet fully developed, but a funding agency is requesting proof of IRB approval? from Yes to No.

- Upon changing the answer from Yes to No, additional questions will need to be completed. Treat the protocol form as if you are creating a new protocol. You can click the Check Validations button to see which mandatory fields need to be completed. If you have questions, contact irbhp@cornell.edu.
- Click on Review and Submit. RASS will ask you to attest by clicking Continue.
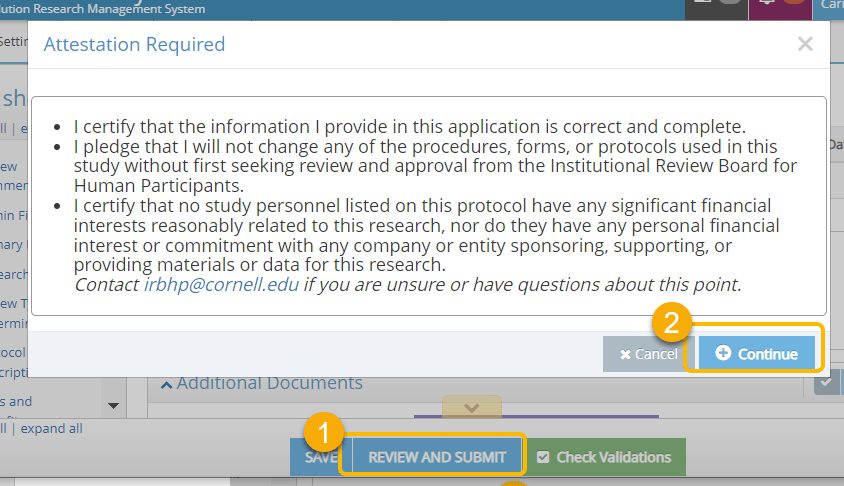
- You now have an opportunity to review your requested changes compared to the previous version of your IRB Protocol. The changes made in the newest version are in the left column, the previous version is in the right. Confirm and Submit the amendment with the changes to the IRB for review or Continue Editing if additional changes are needed prior to completing your submission.

- Your Amendment has been submitted and the protocol will once again be locked for further editing while under review. You will be notified by email if changes are needed following a review.
