Full Board protocols require a continuing review prior to the expiration date. You will receive an email about 60 days from your expiration date requesting that you submit your protocol for review. Click on the link provided in the email to open your protocol or follow the steps below to locate and open your protocol.
- At the top of the screen, use the IRB menu to switch to different screens, including Search IRB Protocols.
- Scroll down the page to the Search Results. You should see a list of protocols in which you are listed as part of the research team.
- You can find information such as the Status of your protocol, Review Type, and Expiration Date directly in the search results.
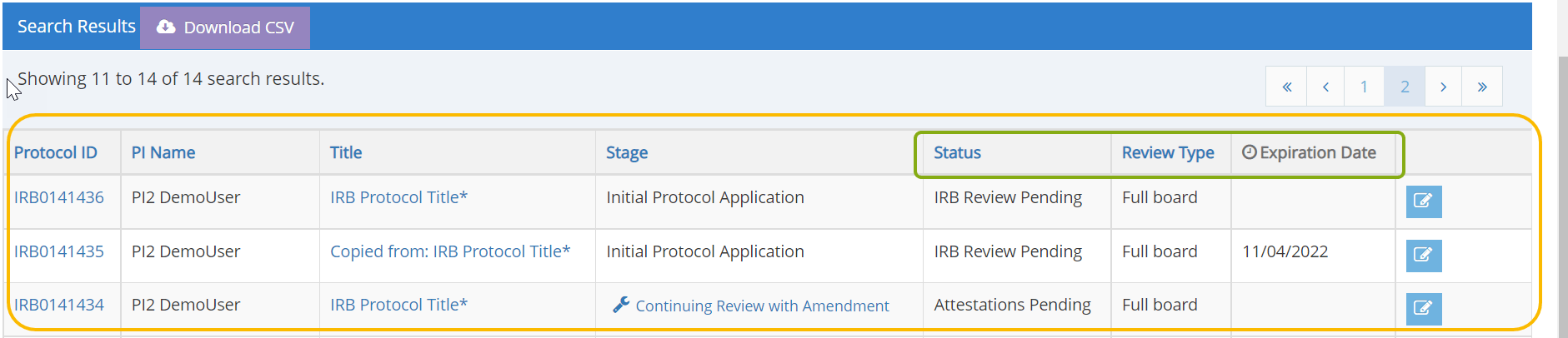
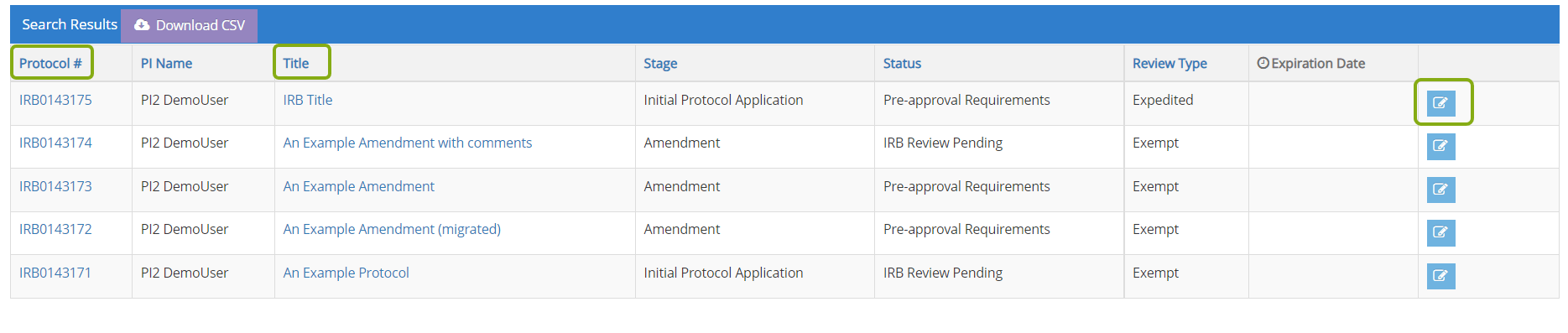
- Locate the protocol you need to submit for continuing review and click on the edit button, protocol ID or title to open it.
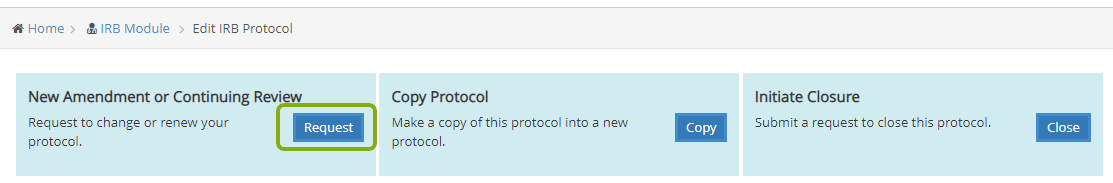
- Once your protocol opens, you will see 3 large blue boxes at the top of the protocol. Locate “New Amendment or Continuing Review” and click the Request button to start the amendment process. Note: If you don’t see these 3 blue boxes, it means you are listed as a research team member on the protocol but do not have permission to edit the protocol. If you need access to edit the protocol, please have your PI send an email to rass@research.cornell.edu requesting your permissions be updated for that protocol.
New Continuing Review Form
- Select the type of Continuing Review if you do not need to request changes to your protocol. Select the type of Continuing Review with Amendment if you need to request changes along with your continuing review.
- If your protocol was approved prior to January 2022 and you have not previously amended the protocol in RASS-IRB, select Continuing Review with Amendment. Some information was imported from Cornell’s old PDF based records but some fields could not be automatically be filled in. You will need to complete all mandatory fields the first time you update the protocol in RASS. You can copy most information from your existing protocol, but a few questions collect additional information. Click the Check Validations button to see which mandatory fields need to be completed. If you have questions, contact irbhp@cornell.edu.
- Complete the requested information and click Save.

- If you chose to amend your protocol along with Continuing Review, make the necessary changes and edits to your protocol. You can use the Panel Shortcuts on the left to get to the specific area of your protocol that require changes. For example, clicking Research Team will take you to the Research Team section so that you can add or remove people from your team. NOTE: If you chose Continuing Review only, you will not be able to edit your protocol.
Continuing Review Section
- Click the Continuing Review section to provide the requested information needed for review. Be sure to check the box to provide your Investigator’s Assurance.
- Click on Review and Submit. RASS will ask you to attest by clicking Continue.
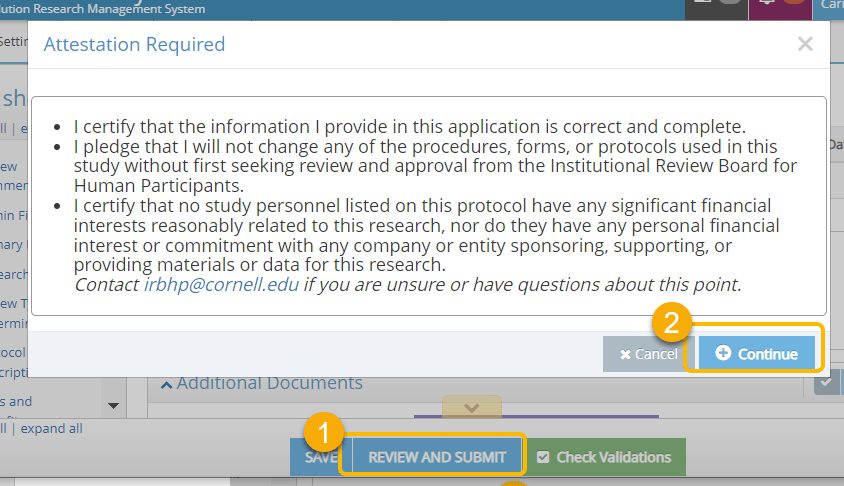
- You now have an opportunity to review your requested changes compared to the previous version of your IRB Protocol. The changes made in the newest version are in the left column, the previous version is in the right. Confirm and Submit the amendment with the changes to the IRB for review or Continue Editing if additional changes are needed prior to completing your submission.
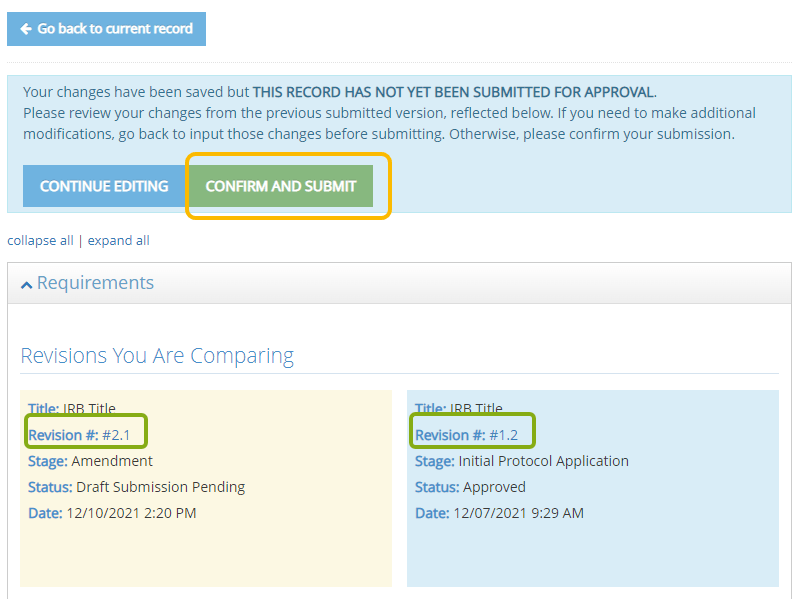
- Your Continuing Review has been submitted and the protocol will once again be locked for further editing while under review. You will be notified by email if changes are needed following a review.
