An unexpected or adverse event that occurs during human participant research should be reported when the investigator becomes aware of the event. Additional information on this policy can be found here. Follow these steps to submit an adverse event to Cornell IRB.
- At the top of the screen, use the IRB menu to switch to different screens, including Search IRB Protocols.
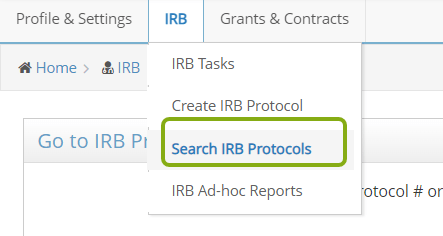
- Scroll down the page to the Search Results. You should see a list of protocols in which you are listed as part of the research team.
- Locate the protocol you need to submit an adverse event for and click on the edit button, protocol ID or title to open it.
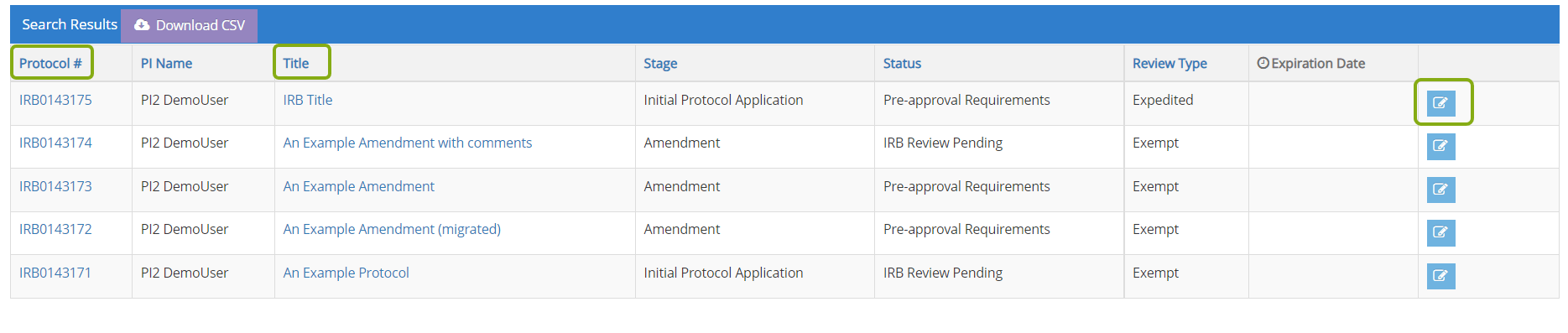
- Once your protocol opens, locate the Adverse Events section from the table of contents. Choose Add Adverse Event to start the process.
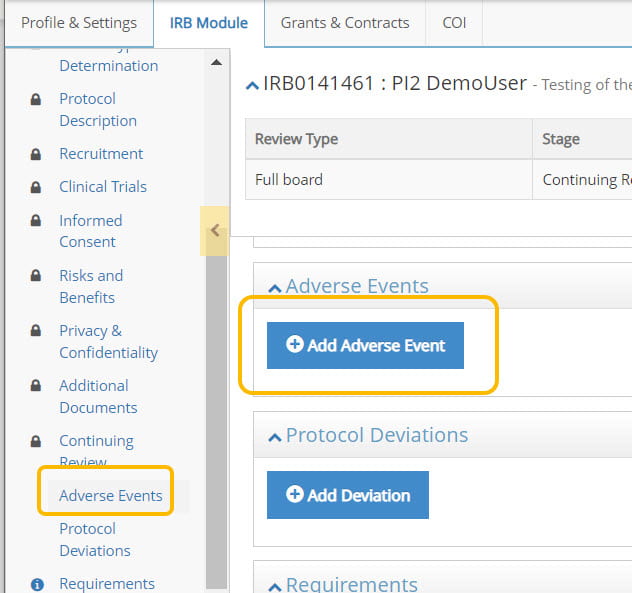
- Complete the adverse event form by providing the information requested in the form. Click Submit and a notification will be sent to Cornell IRB.
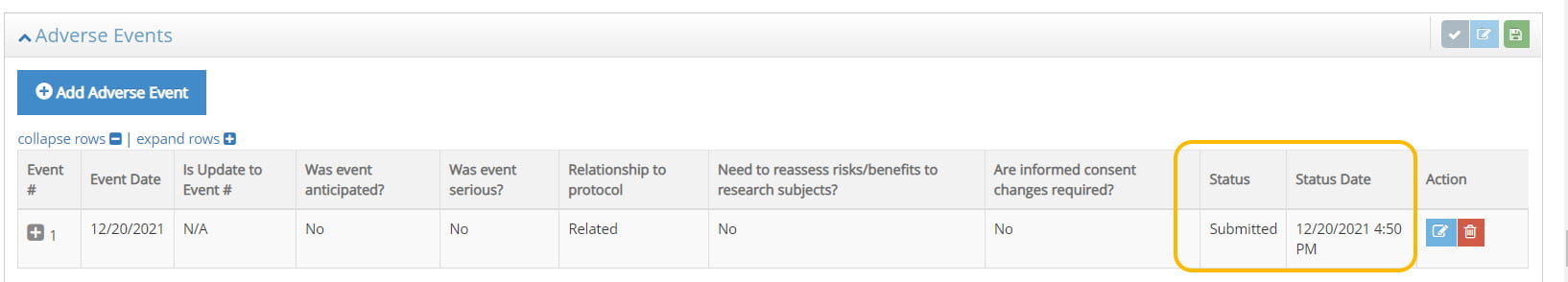
- If additional information is needed, you will receive an email from irbhp@cornell.edu requesting additional information about the adverse event.
