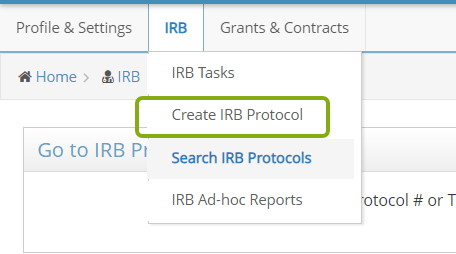
At the top of the screen, use the IRB menu to switch to different screens, including Create IRB Protocol.
 Most of the information you’re asked for should be familiar to you. The following procedure provides more detail for fields that might need explanation.
Most of the information you’re asked for should be familiar to you. The following procedure provides more detail for fields that might need explanation.
As you’re creating your protocol, you can ask for help from the IRB team by contacting irbhp@cornell.edu.
Contents
Start Protocol
- From the IRB menu, select Create IRB Protocol to start a new protocol.
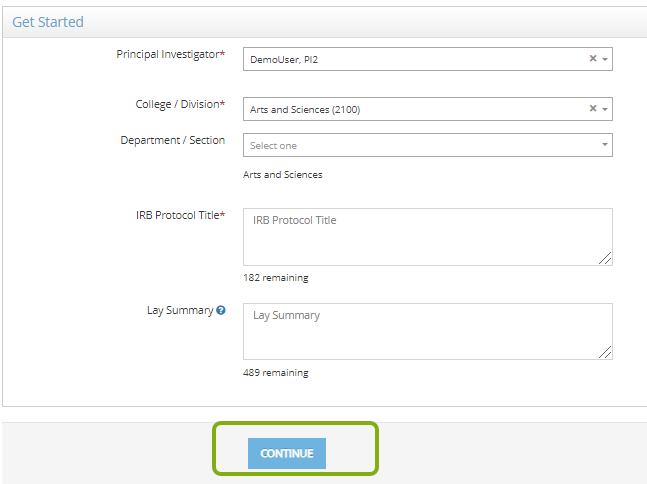
- In the Get Started section, complete the following:
- PI, College/Division, Department/Section: Choose a different PI, if necessary. RASS will fill in the other fields automatically based on information from Workday, if available.
- Faculty Advisor, College/Division, Department/Section: If the PI is a student at Cornell, RASS will ask you to add a faculty advisor. Search for faculty advisor by name or NetID. RASS will fill in the other fields automatically based on information from Workday.
- IRB Protocol Title: This can be changed later.
- Lay Summary: This is optional now but will need to be completed prior to submitting the IRB protocol for review.
- Click Continue.
 When you click Continue, you’ll see the Protocol screen where you can enter the project details. The protocol is assigned an IRB number and is an official Cornell record. You can return to it at any time and can work on it for as long as you need.Tip: Use the Panel shortcuts section at the left to quickly jump from section to section of the protocol.
When you click Continue, you’ll see the Protocol screen where you can enter the project details. The protocol is assigned an IRB number and is an official Cornell record. You can return to it at any time and can work on it for as long as you need.Tip: Use the Panel shortcuts section at the left to quickly jump from section to section of the protocol.
Primary Info
In the Primary Info section, complete the following:
- Type of Project: If this is a student project, add the type of project.
- Indicate if any part of your project is funded by an external sponsor:

- If you do not currently have funding or do no plan to pursue funding, choose No Current Funding.
- If you do not have an OSP number for the funding for this research but are planning to pursue funding in the future, choose Planning to Pursue Funding. You will then be prompted to providing information about the sponsor(s) you intend to request funding for your research.
- If this project is currently funded or you have a proposal for funding, choose Funded/Pending Proposal. If you have an OSP number for your project, you can link it here by clicking on Add Sponsored Research Project.
- A list of your OSP numbers will appear in the Sponsored Project drop-down box. If you are not the PI on the sponsored project, type in the NetID for the PI and the OSP number to locate the project. Save to connect the sponsored project to this IRB protocol.

- A list of your OSP numbers will appear in the Sponsored Project drop-down box. If you are not the PI on the sponsored project, type in the NetID for the PI and the OSP number to locate the project. Save to connect the sponsored project to this IRB protocol.
Research Team
In the Research Team section, the PI is listed along with the person creating the protocol if not the PI. If this is a student protocol, the Faculty Advisor is also listed. You must include all personnel directly involved in this protocol.
- To add another person, click Add Researcher. If you are adding a research team member that is at Weill Cornell or another institution, check the box Cannot find a researcher! Do you want to add an external researcher?
- Assign a Project Role and detail out the Responsibilities for the person you are adding.
- Edit Permission is turned on automatically for the PI, Faculty Advisor or Co-PI. If you’re working collaboratively, only one person can edit the project record at a time. All others will only be able to view. Be sure to log out of the system when you’re finished working to allow your partners to edit.
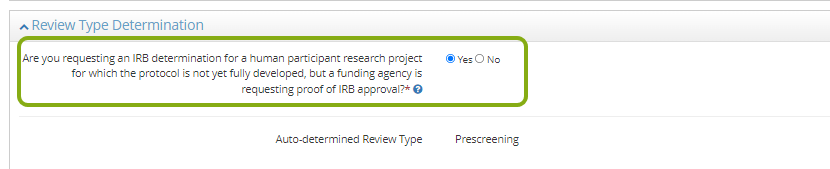
Review Type Determination
In the Review Type Determination section, complete the information for each question. As you complete this section, depending on certain responses you will be prompted to provide more information. Once the section is completed, the system will provide a preliminary determination of the type of review required for your IRB protocol. Please note that this is an auto-determined review type by RASS and is subject to change during the approval process.
If you are requesting a program development protocol for a sponsored proposal, click Yes to Are you requesting determination for a project lacking immediate plans for involvement of human participants, their data, or their specimens (for grant proposals only)?
- RASS will suggest the auto-determined review type of prescreening.
Protocol Description
The Protocol Description section asks you to describe the research and methods as well as provide information about what participants will be asked to do as part of the research. This section is where you indicate if this research will involve the use of secondary data.
- If secondary data will be used as part of this research, click Yes to Will the research involve secondary use of data, documents, records or biospecimens collected from individuals?.
- If this research will only involve the use of secondary data, click Yes to Is the research limited to ONLY the use of secondary use of data, documents, records or biospecimens collected from individuals?
Clinical Trials
The Clinical Trials section asks you a series of questions to determine if your study meets the U.S. government’s definition of a clinical trial. Note: If you indicated your research is limited to secondary data only, you will not see this section.
Additional Documents
Provide any relevant documentation for your protocol in the Additional Documents section.
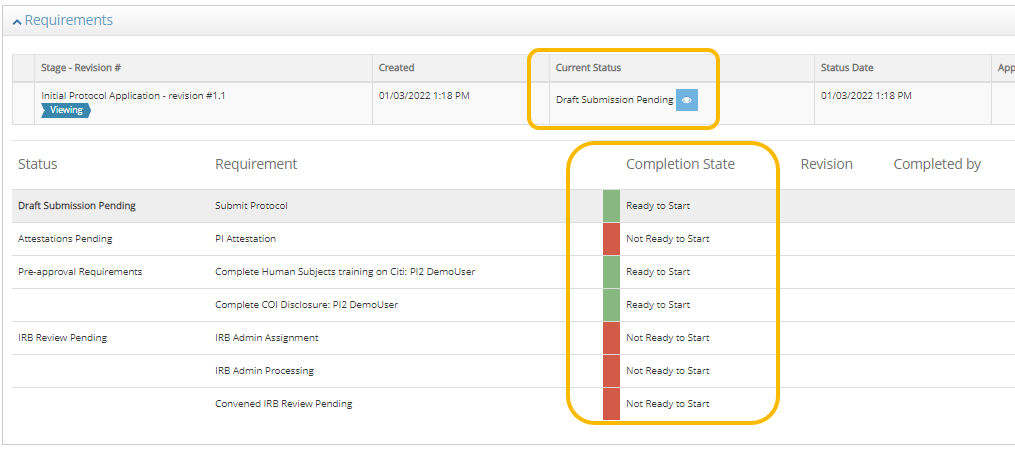
Requirements
Use the Requirements section to track the progress of your protocol as it moves through the approval process.
- The main table lists all requirements and the completion state. If you see Modification Required, hover over the text to see the full note.
- The Stage – Revision table shows the status and approval date. If multiple Revisions of the protocol or project are displayed. Click the magnifying glass to open a previous revision.
Validate Fields and Submit for Review
- At the bottom of the screen, click Check Validations to see errors you need to fix before submitting. You can do this at any time. If you have any missing information on your protocol form, RASS will provide you with links to the missing information as well as indications on the Panel Shortcuts menu.
- When you are ready to submit your protocol to the Cornell IRB for review. Click Submit For Approval.
- If you are the PI listed on the protocol, you will be prompted to provide your Attestation. If you are not the PI, an email will be sent to the PI requesting they provide their attestation.
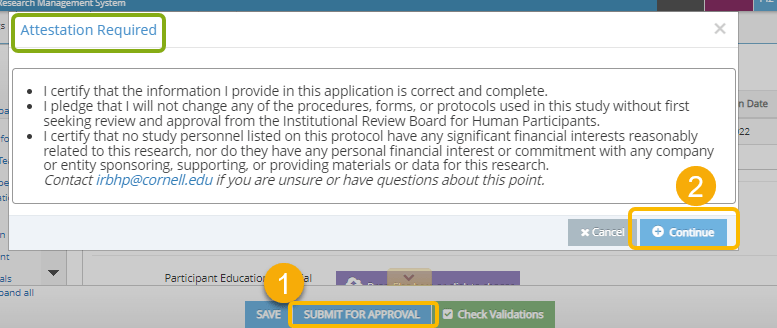
- Read the statement and then click Continue to sign the attestation.
