A protocol is eligible for closure if enrollment of participants is permanently closed; any and all participant interventions and interactions are complete, including collection of long-term follow-up data/samples; no additional identifiable private information will be obtained; and any analysis of identifiable private information is complete. Follow these steps to submit a request to close your protocol to the Cornell IRB.
- At the top of the screen, use the IRB menu to switch to different screens, including Search IRB Protocols.
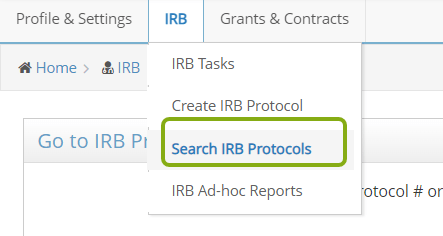
- Scroll down the page to the Search Results. You should see a list of protocols in which you are listed as part of the research team.
- Locate the protocol you need to submit a closure request for and click on the edit button, protocol ID or title to open it.
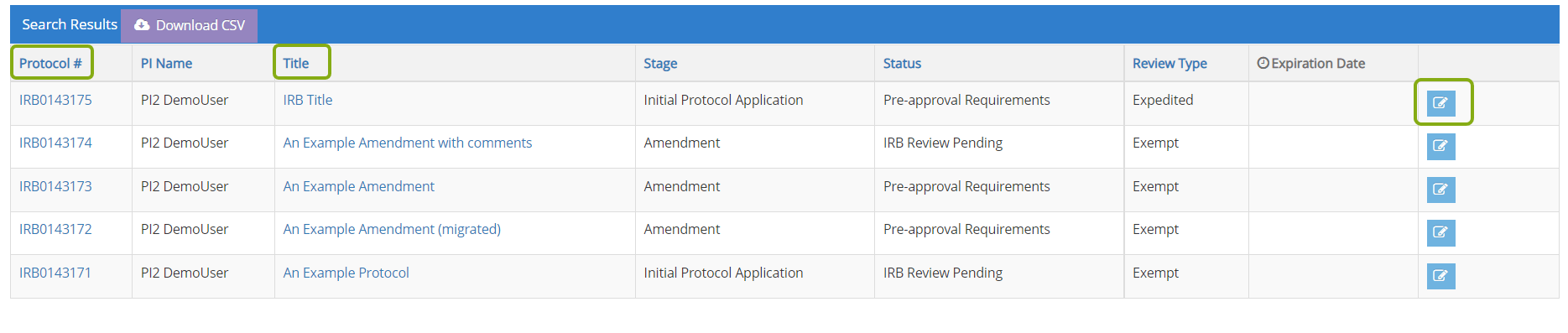
- Once your protocol opens, locate the Initiate Closure blue box at the top of the protocol. Choose Close to start the process.
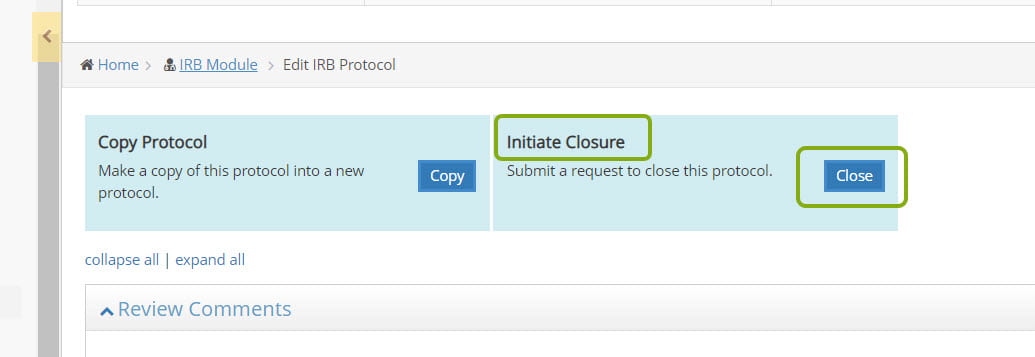
- Complete the closure request and click Save. A notification will be sent to Cornell IRB.
- If additional information is needed, you will receive an email from irbhp@cornell.edu.
- An important note about record retention for closed protocols: Principal Investigators must maintain human participant research records–including signed and dated consent documents, if obtained–for at least three years after completion of a study. Records may need to be kept longer if other regulatory requirements apply. For funded research, confirm the sponsor’s records retention requirements before disposing of human research records. If multiple regulations apply, keep the data for the longest required amount of time. Refer to Cornell’s University Policy 4.21, Research Data Retention for additional information.
